Topical Delivery of Sex Steroid Hormones and Distribution in Different Body Fluids
September 1, 2021
Delivery of the bioidentical hormones estradiol (E2), progesterone (Pg), and testosterone (T) through the skin as a cream or gel (topically) has become a mainstay of bioidentical hormone replacement therapy (BHRT) for women and men. A plethora of FDA-approved pharmaceuticals, compounded, and even over-the-counter topical BHRT products are available. The popularity of these products lies not only in their ease of use, but in proven clinical efficacy [1-5] in treating hormonal deficiencies mostly brought on by aging of the female (menopause) and male (andropause) reproductive systems.
Dosing for Optimal Clinical Benefit
Over the past 30 or so years, higher FDA-approved topical BHRT dosing has dominated allopathic acceptance. However, more practitioners are opening their thinking to alternative views of much lower dosing as being optimal, based on clinical observations coupled with scientific discovery. It also is becoming more evident that while serum and urine testing work fine for detecting endogenously produced hormones, or hormones supplemented by nearly all other forms of delivery (e.g., transdermal patch, oral, vaginal, troche/sublingual, injections and pellets) these body fluids grossly underestimate hormone delivery to capillary beds and hence, tissues, relative to topical delivery [6]. Attempts to achieve physiological levels of E2, Pg, and T in serum with topical BHRT has met with little success, resulting in use of pharmacological dosing to raise serum levels to physiological ranges [6,7].
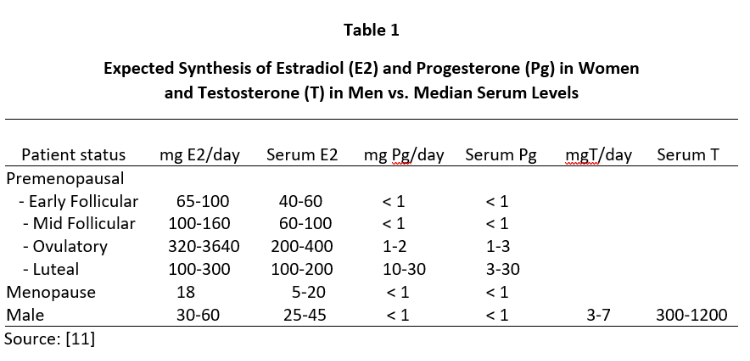
Despite the clear clinical efficacy of topical natural estrogens (mostly E2 and estriol -E3) and Pg in women and T in men, controversy still encircles the dose needed to achieve optimal clinical benefit. This controversy has arisen out of attempts to explain why topical BHRT results in very little increase in venipuncture serum or urine levels of the hormone delivered through the skin [6-8] despite use of topical hormone doses that usually are more than 10x higher than daily endogenous production of these hormones in women and men (see Table 1).
How Does Testing in the Wrong Body Fluid Lead to Overdosing?
Justification for higher dosing of both FDA-approved pharmaceutical and compounded products comes from pharmacokinetic studies showing that pharmacological dosing is necessary to increase serum and urine levels of the dosed hormones to physiological levels. But even with supra-high pharmacological BHRT dosing serum and urine levels of some of the hormones rarely increase to even within low physiological ranges. This is most notable for Pg [6,7,9], and less so for E2 and T [5,10,11] which only increase to physiological levels in serum/plasma and urine with pharmacological dosing.
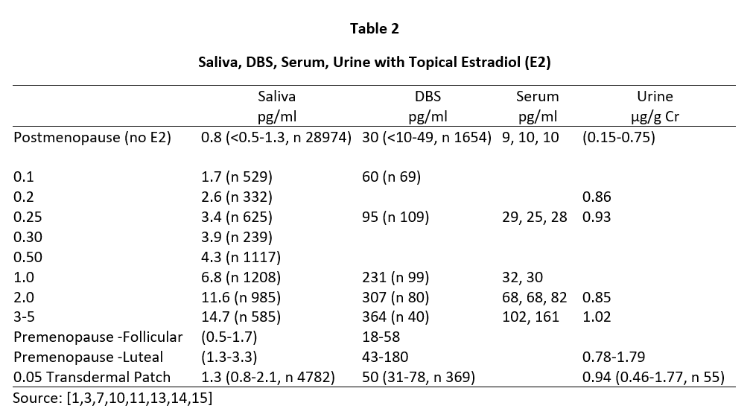
Daily ovarian production of E2 during peak luteal phase of the menstrual cycle is about 100-300 µg [12] when serum and capillary blood levels of E2 are quantitatively equivalent at about 80-200 pg/mL, saliva ranges from about 1.5-4 pg/mL (approximately 2% of serum/plasma levels), and urine levels range from about 0.8-2.0 µg/g Cr (see Tables 2-4). For postmenopausal women to achieve physiological ranges seen in mid-luteal phase of premenopausal women, topical E2 therapy only requires physiological dosing to achieve physiological levels in saliva and capillary whole blood (about 100-300 µg E2), but at least 10x higher dosing (1000-3000 µg = 1-3 mg) to achieve physiological levels in venipuncture serum/plasma and urine (Table 2).
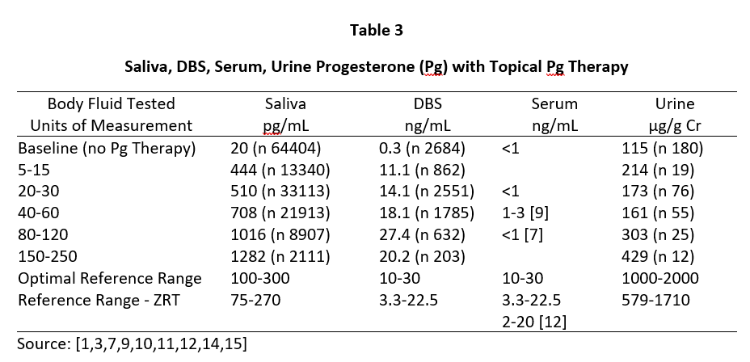
A similar distribution of Pg is seen during peak of the luteal phase of the menstrual cycle where daily Pg production by the ovaries is about 10-40 mg, and the Pg in serum and capillary blood are quantitatively equivalent at about 10-30 ng/mL, saliva about 100-300 pg/mL (approximately 1% of serum/plasma levels), and urine pregnanediol, a surrogate for Pg, about 500-2000 µg/g Cr (Table 3). To achieve premenopausal luteal ranges in postmenopausal women with topical Pg therapy only requires physiological dosing when measuring saliva and capillary whole blood (about 10-50 mg topical Pg) [6,7], but at least 10x higher dosing (100-300 mg topical Pg) when measuring venipuncture serum/plasma. Urine pregnanediol increases very little with increasing doses of Pg [8].
In men, topical T distributes into the same body fluids as topical E2 and Pg in women. In healthy young males, the testes produce about 5-7 mg of T daily and the morning T levels range from about 500-1200 ng/dL in serum/plasma and capillary blood, 100-250 pg/mL in saliva, and 1-4 µg/g Cr in urine (Table 4). To achieve physiological ranges of a healthy young male in the body fluids of hypogonadal men with topical T therapy only requires physiological dosing for saliva and capillary whole blood (about 5-10 mg topical T), but at least 10x higher dosing (50-200 mg topical T) for venipuncture serum/plasma or urine levels.
The lack of increase in serum and urine levels of topically delivered hormones at physiological dosing (50-200 µg E2 and 10-30 mg Pg in women and 5-10 mg T in men) (Tables 2-4) has resulted in justification of dose escalation of FDA-approved and compounded products to amounts that are 10 to 20-fold higher (500-5000 µg E2 and 100-300 mg Pg in women and 50-200 mg T in men) than levels of these hormones that are produced by the gonads of healthy young women and men over a 24-hour period (Table 1). Justification for these 10x pharmacological topical doses is based more on serum and urine levels than clinical response. Only a handful of studies have evaluated the clinical efficacy of lower physiological topical hormone dosing relative to the more allopathic acceptance of pharmacological dosing. However, some studies have clearly shown that more physiological dosing with topical E2 and progesterone are clinically effective for increasing bone mass [3] and protecting the breasts [13] and endometrium [4] at physiological dosing of E2 and Pg applied topically.
Saliva and Capillary Whole Blood Testing More Closely Reflect Tissue Levels
The advent of monitoring steroid hormones in body fluids that more closely reflect tissue levels, namely saliva and capillary whole blood derived from the fingertip (Dried Blood Spot, DBS), has serendipitously shed light on why such high pharmacological dosing is required to achieve even suboptimal physiological levels of hormones in serum and urine. Studies over the past 20 years have revealed that while salivary and capillary blood levels of endogenously-produced steroids quantitatively parallel levels of parent hormones (E2, Pg, T) seen in serum and their metabolites in urine, these equivalencies no longer hold when these steroid hormones are delivered percutaneously as gels or creams. Instead, salivary and DBS levels rise in a more linear fashion with dosing and achieve physiological levels in these body fluids at 10x lower physiological dosing (Tables 2-4); in stark contrast serum and urine levels increase very little at physiological doses.
This has raised the question of why salivary and DBS levels, which are equivalent to free serum levels with endogenously produced hormones, are so much higher (10-100x) than levels seen in serum and urine when hormones are delivered topically. Which one of these categories of body fluids (serum and urine vs. saliva and capillary whole blood) more closely reflect tissue levels of the hormone and the optimal dose that is needed for optimal physiological response?
As a lab that tests all four of these body fluids and is involved in many IRB-approved clinical research projects related to topical BHRT, we have had the opportunity to evaluate levels of endogenous steroids in women and men at different stages of life and supplementing with different hormone delivery systems (oral, occluded patch, topical, vaginal, injections and pellets, troche, etc.) and doses at different time points following hormone application. From these tests and evaluation of symptom relief, we have come to the conclusion that the bioavailable fractions of topically delivered hormones are not accurately quantified by testing serum or urine and that saliva and DBS levels of the topically delivered steroids more closely reflect tissue levels of E2 and Pg in women, and T in men.
Tables 2 and 3 show the median levels of E2 and Pg in saliva, DBS, serum, and urine of postmenopausal women before and after increasing doses of topical E2 and Pg therapies. Table 4 shows the median levels of T in these same body fluids in men over the age of 40 before and after increasing doses of topical T.
Insufficient information was available from our database for serum E2 and Pg (women) and T (men) with different topical doses of these hormones as we test much fewer serum and urine samples than saliva and DBS,; therefore, we used reported median values from several well designed clinical studies that investigated different topical doses and reported serum/plasma levels of each hormone category [1,3,14].
As seen in Tables 2 and 3, physiological luteal levels of E2 and Pg are seen in saliva and capillary blood with physiological topical dosing of E2 (100-300 µg) and Pg (10-30 mg). E2 and Pg in saliva and DBS continue to increase in a somewhat linear fashion with increasing doses. In sharp contrast, the data clearly show that to achieve luteal premenopausal serum levels of estradiol (range 80-200 pg/mL) requires pharmacological dosing (1000-3000 µg) with topical E2, as reported by others [3,14]. Further, physiological luteal levels of Pg in serum and urine are not achieved even with pharmacological (100-300 mg) topical Pg dosing, as reported by others [7]. Dosing at this pharmacological range results in salivary and capillary blood levels of E2 and Pg that are 10-20x higher than physiological range.
As shown in Table 4, topical T in men follows the same pattern as seen with topical E2 and Pg in women in that the physiological levels of T found in young healthy males (Table 1) are seen in the saliva and capillary blood of older hypogonadal men (> 40 y/o) with topical T dosing in a physiological range (5-10 mg). Very little increase in serum and urine T occurs at a physiological topical dose. At least 10-20x higher dosing (50-200 mg) is required to achieve even mid-physiological levels, as reported by others [5]. Dosing at this pharmacological range results in salivary and capillary blood levels of T 10-20x higher than physiological range.
Results from Tables 2-4 clearly show that pharmacological topical dosing with E2 (1000-3000 µg) and Pg (100-300 mg) in women, and T (50-200 mg) in men are required to achieve near physiological mid-luteal levels of serum E2 seen in premenopausal women, and T in healthy young men. In sharp contrast, much lower physiological topical dosing (10-20x lower than pharmacological) is required to achieve optimal physiological levels of these hormones based on saliva and capillary blood levels of E2 and Pg in women, and T in men.
Accurate Testing Leads to Better Dosing Decisions
These data suggest that at least for topical E2 and Pg in women, and topical T in men, serum and urine underestimate by at least 10-fold the amount of topically delivered hormone distributed to several tissues of the body (salivary gland and capillary beds of the finger tip). Similar results have been seen in tissues such as breast biopsies following physiological dosing with topical estradiol (1 mg) and progesterone (25 mg) [13] where mammary tissue levels of hormones increased 100-fold and Pg suppressed E2-stimulated mammary cell proliferation, but serum levels of E2 and Pg showed no significant change from patients treated only with placebo topical cream. These doses of E2 and Pg significantly raise salivary and DBS levels of E2 and Pg to physiological levels but have little impact on serum or urine levels of these hormones. Similar results have been reported for bone response to physiological (250 µg) levels of E2 [3] and Pg (30 mg) to protect the uterus from overstimulation by E2 [4].
Our data show that serum and urine are not appropriate body fluids to monitor topical hormone therapy but are fine for measuring endogenous hormones, or BHRT delivered by other methods.
Saliva or DBS testing provide a more accurate picture of topical doses needed to achieve physiological levels of these hormones in tissues, thus avoiding the potential for overdosing. Continued use of serum or urine for establishing dosing regimens of topically delivered hormones could potentially lead to excessive tissue exposure, suboptimal response, and eventual symptoms/conditions of hormone excess as well as pseudo deficiency caused by tachyphylaxis (down-regulation of tissue receptors and lack of response due to excessive prolonged exposure).
More research is needed to clarify these issues regarding the optimal topical hormone dose necessary to achieve optimal physiological levels of and clinical response to BHRT.
Dr. David Zava Thursday, September 12, 2019
Click here to shop our test kits!
Related Resources
References
[1] Basdevant A and de Lignieres B. Treatment of menopause by topical administration of oestradiol. In: Percutaneous Absorption of Steroids, Mauvais-Jarvis P, Vickers, CFH., and Wepierre J, Eds., Academic Press (London), 1980; pp. 249-258.
[2] Egras AM, Umland EM. The role of transdermal estrogen sprays and estradiol topical emulsion in the management of menopause-associated vasomotor symptoms. Int J Gen Med. 2010;3:147-51.
[3] Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-8.
[4] Leonetti HB, Wilson KJ, Anasti JN. Topical progesterone cream has an antiproliferative effect on estrogen-stimulated endometrium. Fertil Steril. 2003;79:221-2.
[5] Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678-88.
[6] Zava DT, Groves MN, Stanczyk FZ. Percutaneous absorption of progesterone. Maturitas. 2014;77:91-2.
[7] Du JY, Sanchez P, Kim L, Azen CG, Zava DT, Stanczyk FZ. Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum, whole blood, saliva, and capillary blood. Menopause. 2013;20:1169-75.
[8] O’Leary P, Feddema P, Chan K, Taranto M, Smith M, Evans S. Salivary, but not serum or urinary, levels of progesterone are elevated after topical application of progesterone cream to pre- and post-menopausal women. Clin Endocrinol 2000;53:615-620.
[9] Burry KA, Patton PE, Hermsmeyer K. Percutaneous absorption of progesterone in postmenopausal women treated with transdermal estrogen. Am J Obstet Gynecol. 1999;
180:1504-11.
[10] Place VA, Powers M, Darley PE, Schenkel L, Good WR. A double-blind comparative study of Estraderm and Premarin in the amelioration of postmenopausal symptoms. Am J Obstet Gynecol.1985;152:1092-9.
[11] Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA. Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement. Am J Obstet Gynecol. 1985;152:1099-106.
[12] Williams Textbook of Endocrinology, Larsen PR, Kronenberg HM, Melmed S, and Polonsky KS, Eds., 10th Ed., Elsevier Health Services (Saunders), Philadelphia, 2003.
[13] Chang KU, Lee TT, Linares-Cruz G, Fournier S, de Lignieres B. Influence of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo. Fertil Steril 1995;63:785-91.
[14] Brennan JJ, Lu Z, Whitman M, Stafiniak P, van der Hoop RG. Serum concentrations of 17beta-estradiol and estrone after multiple-dose administration of percutaneous estradiol gel in symptomatic menopausal women. Ther Drug Monit. 2001;23:134-8.
[15] Padwick ML, Endacott J, Whitehead MI. Efficacy, acceptability, and metabolic effects of transdermal estradiol in the management of postmenopausal women. Am J Obstet Gynecol. 1985;152:1085-91.